A breakthrough deep-learning model tracks 5,000 fruit fly cells with 90% accuracy, paving the way for early disease detection in human tissues.
- A “Dual-Graph” Innovation: MIT engineers have combined two distinct modeling approaches—”point clouds” and “foams”—to create a unified deep-learning model that captures the complex geometry of developing tissue.
- Unprecedented Accuracy: The model can predict how 5,000 individual cells within a fruit fly embryo will fold, divide, and rearrange with 90% accuracy during the crucial first hour of development.
- Future Medical Applications: While currently used on insects, this technology holds the potential to identify early-onset disease patterns in human organs, such as the specific cell dynamics associated with asthma and cancer.
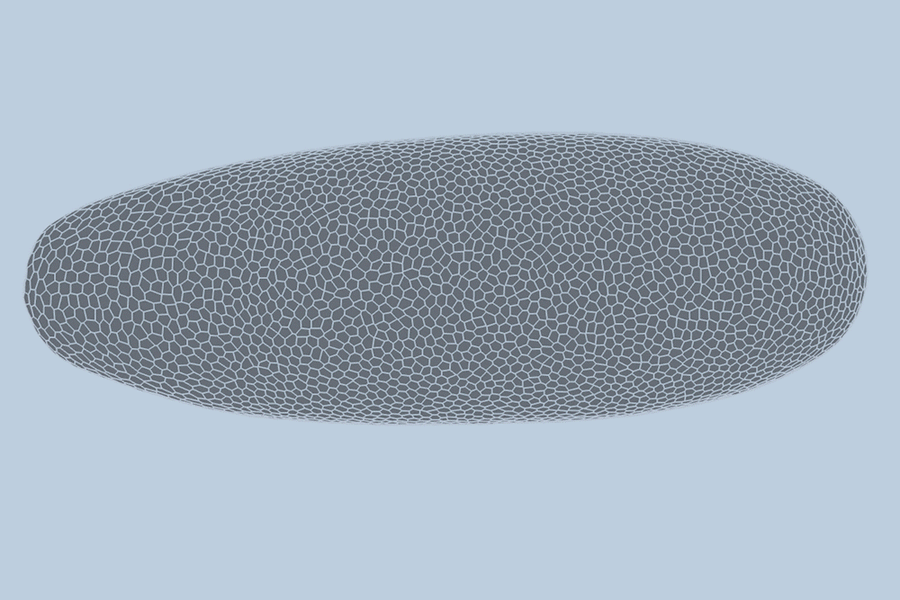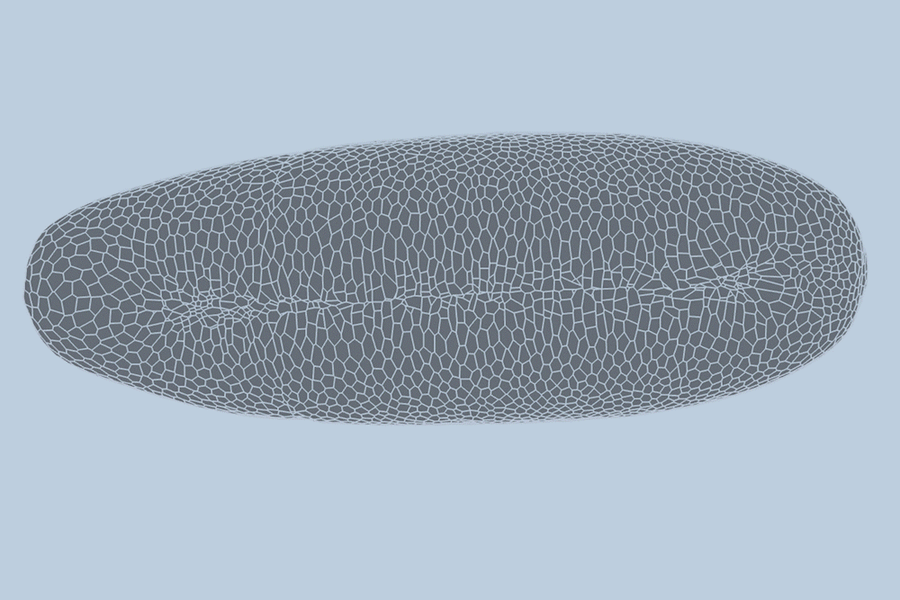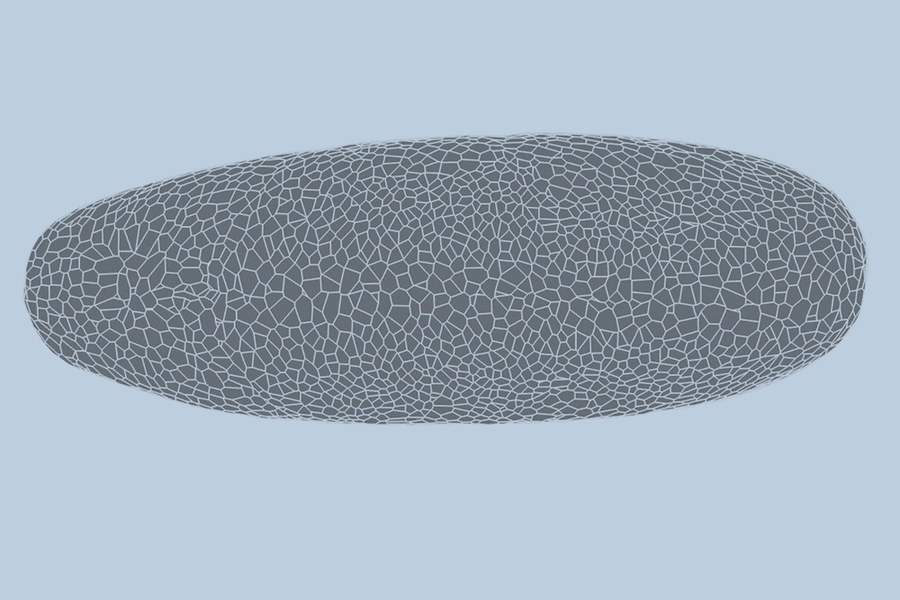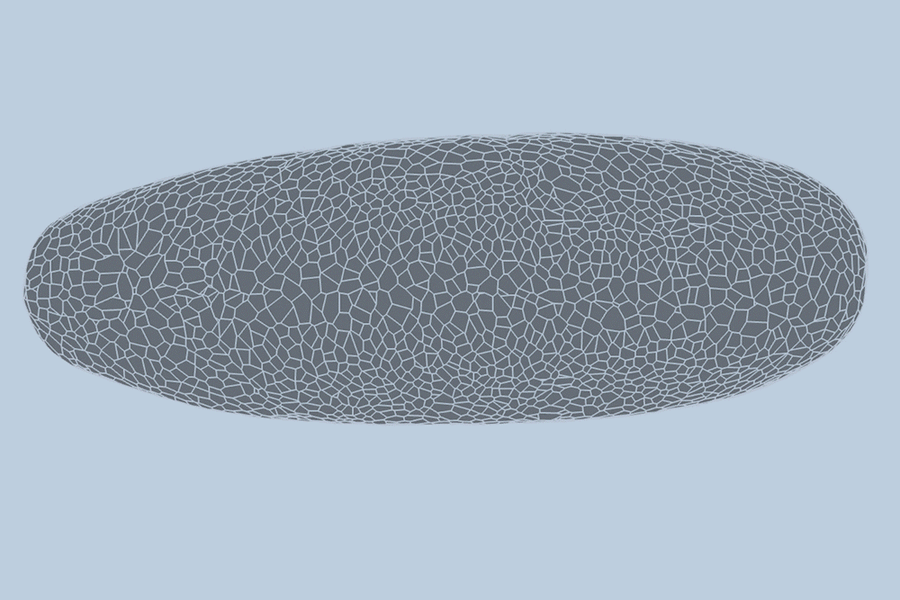
During the earliest stages of life, a biological miracle occurs: a shapeless cluster of cells begins to bloom. Through a complex dance of shifting, splitting, and folding, thousands of cells self-organize to form tissues, organs, and eventually, a living being. For decades, the precise choreography of this process—known as gastrulation—has been difficult to predict.
Now, a team of engineers at MIT has cracked the code. In a study published today in Nature Methods, the researchers present a deep-learning model capable of predicting, minute by minute, how individual cells will rearrange during a fruit fly’s earliest stage of growth. This “MultiCell” method does not just observe; it learns and forecasts geometric changes, offering a proof-of-concept pathway toward a unified map of how life takes shape.
Bridging the Gap: Points Meets Foam
To understand the behavior of a developing embryo, scientists generally rely on one of two modeling methods. The first views the embryo as a “point cloud,” tracking the nucleus of each cell as a dot moving through space. The second views the embryo as a “foam,” where cells are treated like bubbles in shaving cream, emphasizing how they slide and press against one another.
Historically, researchers had to choose a side. However, study author Ming Guo, an associate professor of mechanical engineering at MIT, and graduate student Haiqian Yang decided to embrace both.
“There’s a debate about whether to model as a point cloud or a foam,” Yang explains. “But both of them are essentially different ways of modeling the same underlying graph… By combining these as one graph, we can highlight more structural information, like how cells are connected to each other as they rearrange over time.”
This hybrid approach resulted in a “dual-graph” structure. It captures granular details, such as the location of a cell’s nucleus, while simultaneously mapping the “foam-like” physical connections, such as whether a cell is touching a neighbor or folding at a specific moment.
Predicting the Future, Cell by Cell
To test their model, the team turned to the fruit fly (Drosophila), a standard organism in biological research. Collaborators at the University of Michigan provided high-quality, single-cell resolution videos of fruit fly embryos. These recordings captured the first hour of development—a period of intense activity where the embryo morphs from a smooth, uniform ellipsoid into a structure with defined folds and features.
The team trained their AI on videos of three different embryos, allowing the model to “learn” the rules of cellular interaction. When tested on a fourth, unseen video, the results were staggering. The model predicted the behavior of the embryo’s 5,000 cells with 90 percent accuracy.
“We end up predicting not only whether these things will happen, but also when,” says Guo. “For instance, will this cell detach from this cell seven minutes from now, or eight? We can tell when that will happen.”
From Fruit Flies to Human Health
While predicting the growth of an insect is a scientific triumph, the implications of this research extend far beyond the laboratory. The researchers believe this approach can be applied to more complex organisms, such as zebrafish, mice, and eventually, humans.
The ultimate goal is to use this predictive capability to identify early signs of disease. The team suggests that conditions like cancer and asthma may reveal themselves through specific cellular patterns long before symptoms appear.
“Asthmatic tissues show different cell dynamics when imaged live,” says Yang. “We envision that our model could capture these subtle dynamical differences and provide a more comprehensive representation of tissue behavior, potentially improving diagnostics or drug-screening assays.”
The study, co-authored by Markus Buehler of MIT and researchers from the University of Michigan and Northeastern University, suggests that the only limit to this technology is data.
The “MultiCell” model is ready for broader application; the challenge lies in obtaining high-quality, 3D video data of other tissues. “The real bottleneck is the data,” Guo notes. “If we have good quality data of specific tissues, the model could be directly applied to predict the development of many more structures.”
As imaging technology improves, this AI model could become a standard tool in biology, helping researchers uncover how local cell interactions give rise to global tissues, and perhaps, catching the earliest whispers of disease before they take hold.