Unleashing Scale to Decode Cellular Secrets and Ignite New Treatments
- A New AI Frontier: The launch of C2S-Scale 27B, a 27-billion-parameter model built on Google’s Gemma family, revolutionizes single-cell analysis by treating cells like sentences in a biological language.
- From Hypothesis to Reality: This AI generated a novel prediction for enhancing cancer immunotherapy, pinpointing a drug that amplifies immune signals only in specific tumor environments, validated through lab experiments.
- Scaling for Discovery: By leveraging biological scaling laws, larger models like C2S-Scale don’t just improve on known tasks—they unlock emergent capabilities to hypothesize and explore uncharted cancer pathways, paving the way for innovative therapies.
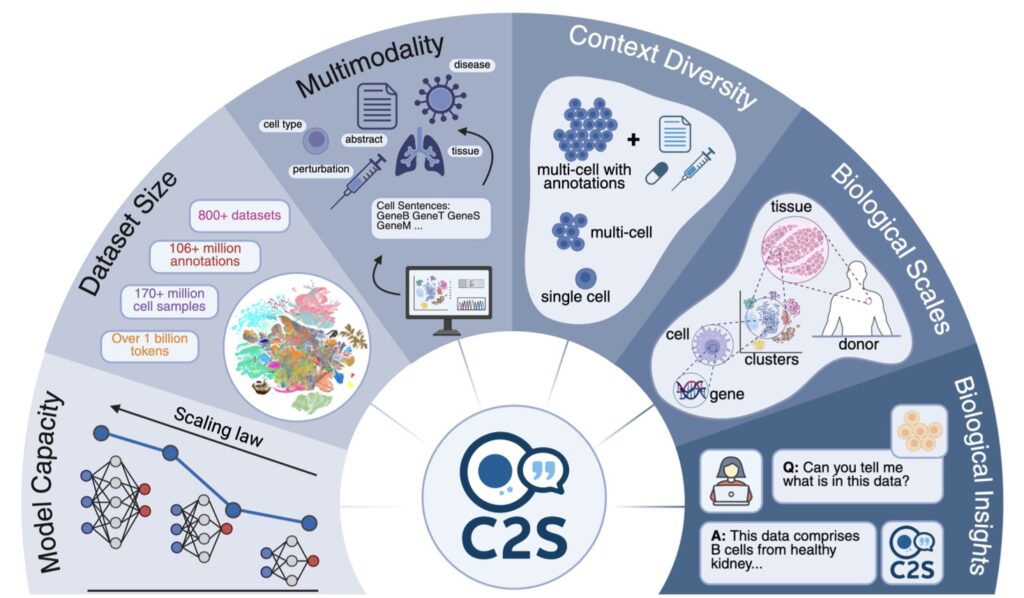
In the rapidly evolving intersection of artificial intelligence and medicine, a groundbreaking achievement is reshaping how we combat cancer. On October 15, 2025, researchers announced the release of Cell2Sentence-Scale 27B (C2S-Scale), a powerful 27-billion-parameter foundation model tailored for single-cell analysis. Developed through a collaboration between AI experts and Yale University, this model builds on the open-source Gemma family from Google, transforming the complex “language” of individual cells into interpretable insights. What makes this launch truly remarkable isn’t just the technology—it’s the real-world impact. C2S-Scale didn’t merely analyze data; it proposed a fresh hypothesis about cancer cellular behavior that has now been experimentally confirmed, revealing a promising pathway for turning “cold” tumors—those evading the immune system—into “hot” ones more susceptible to immunotherapy. This story highlights the transformative potential of AI in science, where scaling up models fosters not incremental gains, but entirely new discoveries.
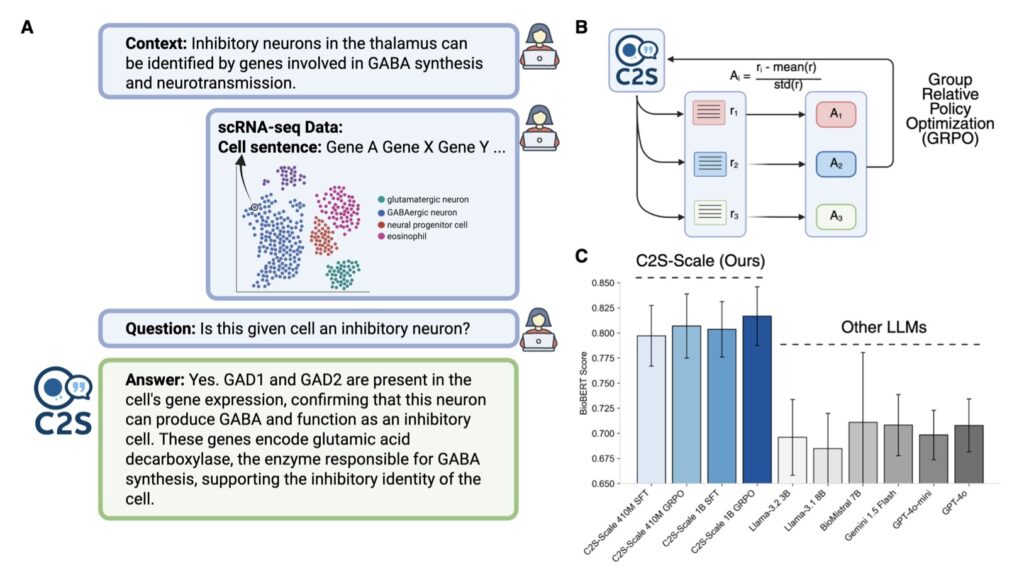
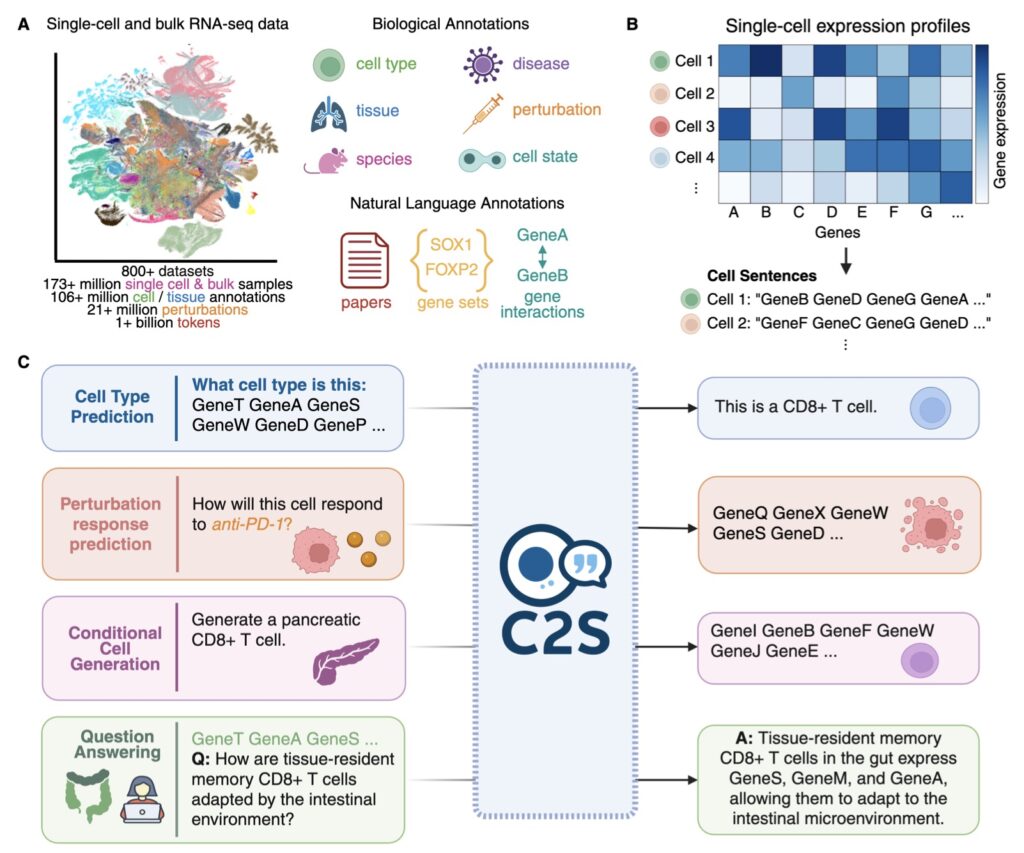
The journey to C2S-Scale stems from a pivotal insight earlier in 2025: biological models, much like those in natural language processing, adhere to clear scaling laws. Just as larger language models grasp nuance and context better, bigger biology-focused AIs excel at decoding intricate cellular interactions. But this raised a profound question—does scaling simply refine performance on familiar tasks, or does it birth novel abilities? The C2S-Scale team pursued the latter, betting that a 27-billion-parameter behemoth could venture into the unknown, generating hypotheses that smaller models couldn’t touch. Trained on vast datasets of single-cell expressions, the model views cells as units in a narrative, enabling it to simulate and predict behaviors in ways that mimic human scientific intuition. This approach addresses a core challenge in cancer research: immunotherapy’s uneven success, particularly against “cold” tumors that fail to signal the immune system effectively through antigen presentation—a process where cells display molecular flags to alert immune defenders.
At the heart of C2S-Scale’s innovation is its ability to perform sophisticated, context-aware reasoning, an emergent trait unlocked by its massive scale. Cancer immunotherapy often falters because many tumors remain stealthy, lacking the interferon-driven signals needed for antigen presentation. The researchers tasked the model with a nuanced virtual screening: identify drugs that act as “conditional amplifiers,” boosting these signals only in immune-context-positive environments—where low interferon levels exist but aren’t sufficient alone—while remaining inert in neutral settings. This demanded a level of conditional logic beyond smaller models’ reach. To execute this, the team devised a dual-context screen. In the first stage, they fed C2S-Scale real-world patient samples featuring intact tumor-immune interactions and subtle interferon activity. In the second, they used isolated cell line data devoid of immune context. The model then evaluated over 4,000 drugs across both scenarios, predicting which would selectively enhance antigen presentation in the patient-relevant, immune-positive context. Strikingly, while 10-30% of its top hits aligned with existing literature, the rest were unexpected gems—drugs with no prior ties to this mechanism—demonstrating the AI’s capacity for genuine innovation rather than rote recall.
One standout prediction from C2S-Scale centered on silmitasertib (CX-4945), a kinase CK2 inhibitor. The model forecasted a sharp “context split”: potent amplification of antigen presentation in immune-context-positive conditions, but negligible impact in neutral ones. This was thrilling because, while CK2’s role in cellular and immune functions was known, no studies had linked silmitasertib specifically to boosting MHC-I expression—the key protein for antigen display. Here, the AI wasn’t parroting facts; it was forging a testable idea from patterns in its training data. To bridge the gap from silicon to biology, the team moved to experimental validation using human neuroendocrine cell models—tissues the model had never encountered during training, ensuring an unbiased test. The results were compelling: silmitasertib alone did nothing to MHC-I levels, and low-dose interferon alone yielded only modest gains. But combined, they synergized dramatically, spiking antigen presentation by about 50%. This synergy made tumors theoretically more visible to immune cells, a critical step toward “heating up” cold ones and enhancing immunotherapy efficacy.
This validation isn’t just a win for C2S-Scale—it’s a blueprint for AI-driven biological discovery. By simulating high-throughput drug screens in silico, the model accelerates hypothesis generation, focusing lab efforts on high-potential leads. It underscores how scaling laws in biology enable context-conditioned insights, like the interferon-conditional effects here, which could inspire combination therapies pairing low-dose signals with targeted inhibitors. Yale teams are now dissecting the underlying mechanisms and probing more AI-suggested predictions across diverse immune scenarios. If preclinical and clinical trials bear fruit, this could hasten new treatments, potentially transforming outcomes for hard-to-treat cancers. Yet, challenges remain: ensuring these hypotheses generalize across patient variability and integrating AI ethically into drug development pipelines.
As C2S-Scale 27B rolls out to the research community today, complete with open resources, it invites global collaboration to decode life’s language further. This isn’t merely a tool—it’s a catalyst for the next era of precision medicine, where AI doesn’t just assist science but co-authors breakthroughs. In a field starved for innovation, such discoveries remind us that the true power of scale lies in illuminating the unseen, one cellular sentence at a time.
